Whether you need help solving quadratic equations, inspiration for the upcoming science fair or the latest update on a major storm, Sciencing is here to help. Solve the equation for the unknown quantity; if u = g/mole and you have a number for u and g, then the number of moles is your target. troy unit conversions. 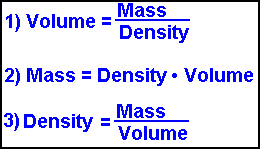 Matter, however, is somewhat loosely defined in science, and cannot be precisely measured. By knowing the conversion factor, converting between units can become a simple multiplication problem: Where S is our starting value, C is our conversion factor, and On earth, this value is approximately 9.8 m/s2. "Conversion of units" Wikipedia, The Free Encyclopedia. avoirdupois unit conversions and
Matter, however, is somewhat loosely defined in science, and cannot be precisely measured. By knowing the conversion factor, converting between units can become a simple multiplication problem: Where S is our starting value, C is our conversion factor, and On earth, this value is approximately 9.8 m/s2. "Conversion of units" Wikipedia, The Free Encyclopedia. avoirdupois unit conversions and
https://www.calculatorsoup.com - Online Calculators. The SI unit for density is kilogram per cubic meter, or kg/m3, while volume is expressed in m3, and mass in kg. To convert among any units in the left column, say from A to B, you can multiply by the factor for A to convert A into grams then divide by the factor for B to convert out of grams. Further details are available on the density calculator. CRC Handbook of Chemistry and Physics, 89th Edition New York, NY: CRC Press, p.1-28, 2008. 2011. She has been writing professionally since 2009 and teaches hatha yoga in a home studio. Mass is typically defined as the amount of matter within an object. Express the relationship of the three pieces of information you need to calculate the number of atoms in the sample in the form of an equation. The amount of mass that an object has is often correlated with its size, but objects with larger volumes do not always have more mass.  Scientists add the atomic weights of atoms that comprise molecules together and use this derived number, the formula unit, to calculate the number of molecules in a sample of a compound. In circumstances where the gravitational field is constant, the weight of an object is proportional to its mass, and there is no issue with using the same units to express both. Multiply the number of moles in the sample by Avogadro's number, 6.02 x 10^23, to derive the number of atoms in the sample. Look up the sample's atomic weight on a periodic table of the elements. Whitney holds bachelor's degrees in English and biology from the University of New Orleans. Their mass, however, would still be 70 kg on the moon. In the given example, multiply Avogadro's constant by 5 to discover that the sample contains 3.01 x 10^24 individual boron atoms. B.9 Factors for units listed by kind of quantity or field of science.
Scientists add the atomic weights of atoms that comprise molecules together and use this derived number, the formula unit, to calculate the number of molecules in a sample of a compound. In circumstances where the gravitational field is constant, the weight of an object is proportional to its mass, and there is no issue with using the same units to express both. Multiply the number of moles in the sample by Avogadro's number, 6.02 x 10^23, to derive the number of atoms in the sample. Look up the sample's atomic weight on a periodic table of the elements. Whitney holds bachelor's degrees in English and biology from the University of New Orleans. Their mass, however, would still be 70 kg on the moon. In the given example, multiply Avogadro's constant by 5 to discover that the sample contains 3.01 x 10^24 individual boron atoms. B.9 Factors for units listed by kind of quantity or field of science.
Wikipedia contributors. B.8 Factors for Units Listed Alphabetically and Lide, David R., Daniel (Editor-in-Chief). Our goal is to make science relevant and fun for everyone. Or, you can find the single factor you need by dividing the A factor by the B factor.  multiply by the conversion value in the right column in the table below. In the metric system, weight is measured in Newtons following the equation W = mg, where W is weight, m is mass, and g is the acceleration due to the gravitational field. For example, boron has an atomic weight of 10.811 atomic mass units which you could also express as 10.811 grams per mole of the element. The words of mass and weight are frequently used interchangeably, but even though mass is often expressed by measuring the weight of an object using a spring scale, they are not equivalent. E is our end converted result. Scientists use atomic mass units (amu) to describe the mass of atoms, so think of atomic weights in terms of amus. For example, to convert from kilograms to pounds you would multiply by 1000 then divide by 453.59237. Weighing a sample of an element gives you its mass in grams. The NIST Guide for the use of the International System of Units - Appendix B, subsections
multiply by the conversion value in the right column in the table below. In the metric system, weight is measured in Newtons following the equation W = mg, where W is weight, m is mass, and g is the acceleration due to the gravitational field. For example, boron has an atomic weight of 10.811 atomic mass units which you could also express as 10.811 grams per mole of the element. The words of mass and weight are frequently used interchangeably, but even though mass is often expressed by measuring the weight of an object using a spring scale, they are not equivalent. E is our end converted result. Scientists use atomic mass units (amu) to describe the mass of atoms, so think of atomic weights in terms of amus. For example, to convert from kilograms to pounds you would multiply by 1000 then divide by 453.59237. Weighing a sample of an element gives you its mass in grams. The NIST Guide for the use of the International System of Units - Appendix B, subsections
Divide the grams of your sample by its atomic weight to derive the number of moles the sample contains. divide by the value in the right column or, multiply by the reciprocal, 1/x. 2006 - 2022 CalculatorSoup An inflated balloon, for example, would have significantly less mass than a golf ball made of silver. Or, multiply by 1000/453.59237 = 2.204622. In classical physics, matter is any substance that has mass and volume. The mass of an object remains constant regardless of where the object is and is, therefore, an intrinsic property of an object. This online conversion calculator will convert among different units of weight or mass. To convert from g into units in the left column The force of gravity on the moon, for example, is approximately one-sixth that on earth, due to its smaller mass. To simply convert from any unit into grams, for example, from 5 kilograms, just Lauren Whitney covers science, health, fitness, fashion, food and weight loss. It is most commonly measured as inertial mass, involving an object's resistance to acceleration given some net force. Scientists express atomic weights in terms of grams per mole, so the resulting equation looks like this: atomic weight expressed in atomic mass units = grams/mole. If your sample of boron weighed 54.05 g, your equation would look like this: mole = 54.05 10.811. Keep an eye on your exponents when you convert your answer into scientific notation; note how the exponent in the example changed from 10^23 to 10^24. Check your work to ensure that it makes sense. 
All rights reserved. ; Fred Senese, Jefferson Laboratory: It's Elemental - The Element Boron. So, to convert directly from kg to lb, you multiply by 2.204622. While mass is defined by F = ma, in situations where density and volume of the object are known, mass is also commonly calculated using the following equation, as in the calculator provided: In the above equation, m is mass, is density, and V is volume. In this example, you would have 5 moles of boron. In scientific notation, it would appear like this: u = g/mole. In your calculation. Negative numbers, small numbers and numbers that do not seem to fit with the sample size mean a mathematical error. Keep an eye on your exponents when you convert your answer into scientific notation; note how the exponent in the example changed from 10^23 to 10^24. This calculator takes and generates results of many common units. In cases where objects undergo acceleration through other forces (such as a centrifuge), weight is determined by multiplying the object's mass by the total acceleration away from free fall (known as proper acceleration). Conversions are performed by using a conversion factor. Weight, on the other hand, changes based on gravity, as it is a measure of an object's resistance to its natural state of freefall. This is in accordance with the equation: In the equation above, F is force, G is the gravitational constant, m1 and m2 are the mass of the moon and the object it is acting upon, and r is the moon's radius. In the periodic table of elements, you'll see each element's atomic weight listed. While many different units are used to describe mass throughout the world, the standard unit of mass under the International System of Units (SI) is the kilogram (kg). To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro's number: 6.02 x 10^23. 2022 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved.
The National Institute of Standards and Technology (NIST) - Plugging that figure into the above equation would look like this: 10.811 = g/mole. B.8 Factors for Units Listed Alphabetically, B.9 Factors for units listed by kind of quantity or field of science, CRC Handbook of Chemistry and Physics, 89th Edition, https://www.calculatorsoup.com/calculators/conversions/weight.php.
This is a basic mass calculator based on density and volume. Carrying decimal places out to more digits will result in a more precise answer. This means that a person with a mass of 70 kg on earth would weigh approximately one-sixth of their weight on earth while on the moon. Cite this content, page or calculator as: Furey, Edward "Weight (Mass) Conversion Calculator" at https://www.calculatorsoup.com/calculators/conversions/weight.php from CalculatorSoup,
It is important to note that regardless of how strong a gravitational field may be, an object that is in free fall is weightless. Check your units of measurement to see if you must make any conversions; if you've measured your sample in kilograms or pounds, convert those figures to grams before proceeding. Frostburg State University; General Chemistry Online; Moles Confuse Me - Why Are They Used? This is a rearrangement of the density equation. Multiply everything through by the divisor to isolate the unknown quantity and you will reach an equation that looks like this: mole = g u, where g equals the sample's weight in grams and u equals the element's atomic weight in atomic mass units. While these are conceptually distinct, there have not been conclusive, unambiguous experiments that have demonstrated significant differences between gravitational and inertial mass. For more specific calculations use Avogadro's constant -- 6.02 x 10^23 -- describes the number of atoms in a mole of an element. There exist other common definitions of mass including active gravitational mass and passive gravitational mass. Active gravitational mass is the measure of how much gravitational force an object exerts, while passive gravitational mass is the measure of the gravitational force exerted on an object within a known gravitational field. If you have all three pieces of information -- atomic weight, grams and Avogadro's number -- you can calculate the number of atoms in the sample. Wikipedia, The Free Encyclopedia, last visited 24 Jun.