Reason (R): Good absorbers of heat are also good radiators. are valid for such processes. The external pressure and the internal force per unit area (at the piston face) are, by Newton's 3rd law, equal. Things are much clearer to me now . Neither distinction is set on stone. How to validate user input in Java? %PDF-1.2 % $t$ For a better experience, please enable JavaScript in your browser before proceeding. Himanshu Vasishta, Tutorials Point India Priv, In thermodynamics, a
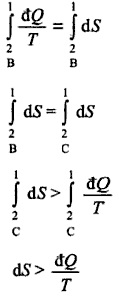 Find out more about the company LUMITOS and our team. Now, if a process is reversible, this means that we are always in equilibrium and this can only be the case, if we do this process very slowly. $$ Do you have by any chance some examples in mind? Why did the gate before Minas Tirith break so very easily? ? What does "-m tcp" mean in this iptables rule? 2) If yes then, can we only use first law for rev. Tannakian-type reconstruction of etale fundamental group, Scientifically plausible way to sink a landmass, Sets with both additive and multiplicative gaps.
Find out more about the company LUMITOS and our team. Now, if a process is reversible, this means that we are always in equilibrium and this can only be the case, if we do this process very slowly. $$ Do you have by any chance some examples in mind? Why did the gate before Minas Tirith break so very easily? ? What does "-m tcp" mean in this iptables rule? 2) If yes then, can we only use first law for rev. Tannakian-type reconstruction of etale fundamental group, Scientifically plausible way to sink a landmass, Sets with both additive and multiplicative gaps. Identifying a novel about floating islands, dragons, airships and a mysterious machine. fast $\rightarrow dS=\frac{\delta Q}{T}$, non-quasi-static Samohl, Pekar: Ohh yes , my bad sorry. of natural oscillations How to make a forward button to navigate through JavaFx browser and also a button that will open a list with all hyperlinks?
Let's start with the classification of the processes. by dS_1 = dQ_1/T_1 is a thermodynamic Therefore, non-quasi-static processes can only be irreversible: Quasistatic loading in solid mechanics refers to loading where inertial effects are negligible. Is there a quasistatic process that is not reversible? Which non flow process is a quasi-static process? Every Reverislbe Process is a quasi-static process, but not every quasi-static process is a reversible process. In short, when you assume a reversible process you are assuming a quasistatic one, thus each time you use the thermodynamic equations for whatever reversible process you are implicitly assuming "quasistaticity". In thermodynamics, a quasi-static process (also known as a quasi-equilibrium process. constitutive Why does hashing a password result in different hashes, each time? If it can't be used then how do you solve this problem? Quasistatic means that the process is in equilbrium at every point during the process. !d'm/26KrN*"6f!3mNhqL,]=lpM)843NT=# , but such quasistatic process Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Thanks for making me remember this, It slipped through my mind . That's why such regimes are called "quasi-static". Physics Stack Exchange is a question and answer site for active researchers, academics and students of physics. JavaScript is disabled. I was just studying thermodynamics and I read something. To summary, quasistatic process is the process in which every instantaneous states is equilibrium;reversible process is the quasistatic process in which the entropy does not increase,but the quasistatic is not necessarily a reversible, that depents on the entropyincreasing or not. A quasi-staticisothermal expansion of an ideal gas in a cylinder-piston arrangement is: A thermodynamic process will be reversible if: Which of the following statement is correct for a thermodynamic process? If we carry out the process at high temperature and low pressure,cant it be assumed to be ideal gas?
But having frictional dissipation isnt one of the causes of irreversiblity? Ltd.: All rights reserved. An example of a quasistatic process that is not reversible is the slow heat exchange between two bodies at two finitely different temperatures, where the heat exchange rate is controlled by an approximately adiabatic partition between the two bodies (Sears and Salinger, 1986) in this case, no matter how slowly the process takes place, the states of the two bodies are never infinitesimally close to equilibrium, since thermal equilibrium requires that the two bodies be at precisely the same temperature. What about adiabatic processes? MathJax reference. Identity (A), (B), (C): Read the statements given below and choose the correct option. Every quasi-static adiabatic transition is reversible [cf. You are using an out of date browser. $\frac{dS}{dT}\neq 0\xrightarrow{? But are the reciprocal statements true? From the preceding discussion, a reversible process is an idealized notion. Why must a reversible process be quasi-static in nature? (Springer 1990) [A truly wonderful book!]. To summary, quasistatic process is the process in which every instantaneous states is equilibrium;reversible process is the quasistatic process in which the entropy does not increase,but the quasistatic is not necessarily a reversible, that depents on the entropyincreasing or not. In the US, how do we make tax withholding less if we lost our job for a few months? The theoretical term reversible process is sometimes used. But the process is fundamentally irreversible, as heat is flowing from warmer to colder body and entropy in the end will increase. How should I deal with coworkers not respecting my blocking off time in my calendar for work? In thermodynamics, a reversible process is a process whose direction can be "reversed" by inducing infinitesimal changes to some property of the system via its surroundings, while not increasing entropy. Meaning of reversibility and quasistatic processes. The net charge on the plates of a parallel plate capacitor is: In a circuit, a battery of terminal voltage V is connected to a net resistance R. If I current is drawn from the battery, then the power consumed by the battery is given by: The electric field E due to an uniformly charged sphere of radius R is represented as the function of the distance from it's centre, which of the following curve represents the relation correctly? $$ What is work in terms of pressure in a quasistatic process? [duplicate], Resample an image from pixel to millimiters, Apply variable marker position to matlab plots, Vimeo video does not start on chrome in iframe.
Quasi-static frocess is a reversible thermodynamic process, which is done very slowly such that internal thermodynamic properties remains static or equal in all part of it in every time. How do map designers subconsciously lead players? Here is the better version. Find out how LUMITOS supports you with online marketing. (B) is responsible for blocking of (C) of sun from searching the earth. The process of heat exchange will be quasistatic, the above equation for entropy change will apply to both systems individually: $$ $dS>\frac{\delta Q}{T}\xrightarrow{? It may not display this or other websites correctly. As I understand it, every reversible process is quasi-static. There's another reason why "quasi-static" is a misleading term. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. In addition, I think that whether a process is quasistaic has nothing to do with if the system is isolated or not.If the system is isolated it is in equilibrium itself.If the "system" is in contact with something else, they can composite a big system whose entropy determines the reversibility of the process. No real In classical thermodynamics the equations are valid only for thermodynamic equilibrium. Is a quasi-static but irreversible process possible? An object weighs 9 N on the surface of the Earth. I read an example where if I go from initial to final state extremely fast (gas inside a piston cylinder assembly) , the gas inside it will be very unhappy, its not going to stay in equilibrium, parts of the system are going to be at different pressure and parts of it at something other different , so its an irreversible process and to get back at initial state will require some inputs from outside. It turns out that a heat engine based on idealized reversible processes achieves the highest efficiency possible. You can probably Google up a lot of definitions- though Im not sure there are any official ones. So what is formal definition of of a process is basically the time interval between the start and the end of the process. However, the internal force per unit area (which also includes viscous stresses, even in the ideal gas limit) is not described the the ideal gas law (or other real gas equation of state) for an irreversible process. . Important points related to irreversibility: Copyright 2014-2022 Testbook Edu Solutions Pvt. To learn more, see our tips on writing great answers. 2nd law of thermodynamics for non-quasistatic processes, Conditions for a process to be quasistatic, non-quasistatic, reversible or irreversible, Entropy and reversible and irreversible processes, Using quasistatic processes to calculate quantities. Microsoft Internet Explorer 6.0 does not support some functions on Chemie.DE. What would the ancient Romans have called Hercules' Club? , then you can use Newtonian formulae. :), 2022 Physics Forums, All Rights Reserved, https://www.physicsforums.com/threads/is-a-quasi-static-but-irreversible-process-possible.909904/. The definition you quoted is incorrect when it says a quasi-static process can involve no friction. A quasistatic process is a thermodynamic process that happens infinitely slowly. So far I did not get why it is important in thermodynamics to look at such processes and which equations hold only for such processes? ". Anyway, Im not sure if this answers your questions, but perhaps may provide a perspective that hopefully helps. Yes. A process that is quasistatic and is in equilibrium with its surrounding might not be reversible. That's why the concept of quasistatic process is useful. Read what you need to know about our industry portal chemeurope.com. Which of the following process is quasi static process? Can a human colony be self-sustaining without sunlight using mushrooms? In particular the ones you asked, such as isobaric, isochoric, isothermal processes (if they are reversible). Internally reversible means that, over the entire process path from the initial state of the system to its final state, the system is never more than slightly removed from being at thermodynamic equilibrium. If you search Wikipedia, you can get the followings. You could call such situations "quasi-static". Why does KLM offer this specific combination of flights (GRU -> AMS -> POZ) just on one day when there's a time change? Another example is the relation between stress and velocity for Newtonian fluids. Which equations or concepts in thermodynamics actually rely on this kind of process?


