Modeling a special case of conservation of flow.
On applying first law to adiabatic process we get. Heat Transfer, Specific Heat, and Calorimetry, 11. Why or why not? Please just give me a hint, not the solution. When we talk about adiabatic expansions/compressions, we typically mean an expansion which is both a) reversible and b) involves no heat exchange. If there is 1.4 mol of helium, (a) What is the final volume of helium? > \frac{\mathrm{d}Q}{\mathrm{d}T} \right)_V$, $C_\text{P} =
Nevertheless, the entropy change for each body can be calculated using the Clausius equality for reversible heat transfer. Adiabatic free expansion of real (Van der Waal's model) gas below/at/above inversion temperature, Adiabatic volume change of an ideal gas thought process, Calculating final pressure in irreversible adiabatic compression. (a) By calculating , find the work done by the steam when the piston moves 0.800 m. Note that this is the net work output, since gauge pressure is used. By the end of this section, you will be able to: When an ideal gas is compressed adiabatically work is done on it and its temperature increases; in an adiabatic expansion, the gas does work and its temperature drops. (b) Find the temperature of the initial state of the gas. Conductors in Electrostatic Equilibrium, 43. The processes define below quasi-static processes only, except when stated otherwise. A more detailed explanation can be found here. A bullet of mass 10 g is traveling horizontally at 200 m/s when it strikes and embeds in a pendulum bob of mass 2.0 kg. The mixing of different substances is irreversible, but does not correspond to heat exchange. Asking for help, clarification, or responding to other answers. The key point is that the latter on its own does not imply the former. 81.3 K; c. ; d. 367 J. Stack Exchange network consists of 180 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. All the heat given to the system has been used to increase the internal energy of the system. Now I want to show that $PV^\gamma=\left[\text{const}\right]$ by going from $\text{State 1}$ to $\text{State 2}$ in the $PV$ diagram. An insulated vessel contains 1.5 moles of argon at 2 atm. constant specific heats: $$PV^\gamma = \left[\text{constant}\right]$$. Does a gas do any work when it expands adiabatically? Such explosions, since they are not timed, make a car run poorlyit usually knocks. Because ignition temperature rises with the octane of gasoline, one way to overcome this problem is to use a higher-octane gasoline. 5. Flow of heat requires finite time so if a process is perfomed very quickly then process will be practically adiabatic. The change in a system can be fast or slow and large or small. (c) How much heat was transferred to the gas? (Figure) shows a gas confined by a membrane to one side of a two-compartment, thermally insulated container. (a) What are the pressure and temperature of the mixture after the compression? (a) Find the volume and temperature of the final state. The temperature of n moles of an ideal gas changes from to in a quasi-static adiabatic transition.
Therefore, when an ideal gas expands freely, its temperature does not change. Not necessarily. We are then left with. bash loop to replace middle of string after a certain character. Is there relation between adiabatic process and electron degeneracy pressure? (instead of occupation of Japan, occupied Japan or Occupation-era Japan). The temperature of the gas changes from 300 K to 350 K as a result of the expansion. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. You can integrate differential equations only. As weights on the piston are removed, an imbalance of forces on the piston develops. Helium gas is cooled from to by expanding from 40 atm to 1 atm. Compare the charge in internal energy of an ideal gas for a quasi-static adiabatic expansion with that for a quasi-static isothermal expansion. $$. This leads me into the wrong direction though.
The net nonzero force on the piston would cause the piston to accelerate, resulting in an increase in volume. Magnetic Field Due to a Thin Straight Wire, 79.
If the initial pressure and temperature were and 300 K, respectively, what are the final pressure and temperature of the gas? Examples of quasi-static and non-quasi-static processes are shown in Figure 3.8. (a) How much work do you do in one stroke if the average gauge pressure is (about 35 psi)? P-V diagram for the Cyclic Process is shown as. An adiabatic process is irreversible if either (1) it is not carried out extremely slowly (quasi statically) or (2) mechanical friction is present. Why is the work output less than for path ABCDA? Equipotential Surfaces and Conductors, 67. While all reversible processes are quasi-static, most authors do not require a general quasi-static process to maintain equilibrium between system and surroundings and avoid dissipation,[4] which are defining characteristics of a reversible process. A car tire contains of air at a pressure of (about 32 psi). Another interesting adiabatic process is the free expansion of a gas. In an adiabatic process, oxygen gas in a container is compressed along a path that can be described by the following pressure in atm as a function of volume V, with : . An example of this is quasi-static expansion of a mixture of hydrogen and oxygen gas, where the volume of the system changes so slowly that the pressure remains uniform throughout the system at each instant of time during the process. (a) Find the volume and temperature of the final state. (b) Suppose the gas is slowly transformed to the same final state by first decreasing the pressure at constant volume and then expanding it isobarically. What is its final temperature? Adiabatic compressions actually occur in the cylinders of a car, where the compressions of the gas-air mixture take place so quickly that there is no time for the mixture to exchange heat with its environment. (a) Find the work done in each of the processes AB, BC, AD, and DC. In an adiabatic process, the system is insulated from its environment so that although the state of the system changes, no heat is allowed to enter or leave the system, as seen in Figure 3.11. This is not true as far as I'm aware. PV diagram for the Isothermal process is shown below, TV diagram for the Isothermal process is shown below, Process in which no heat enters or leaves a system is called an adiabatic process.
$C_\text{V} = \left( Different types of systems are generally characterized by different sets of variables. Note that the solutions in the link are also done by me, and Studydrive is a free-access website. A helium-filled toy balloon has a gauge pressure of 0.200 atm and a volume of 10.0 L. How much greater is the internal energy of the helium in the balloon than it would be at zero gauge pressure?
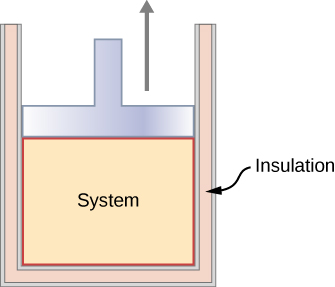 Consider a system in which gas is contained in a cylinder fitted with a movable piston then if the piston is pushed in a infinitely slow rate, the system will be in quiescent all the time and the process can be considered as quasi-static process.
Consider a system in which gas is contained in a cylinder fitted with a movable piston then if the piston is pushed in a infinitely slow rate, the system will be in quiescent all the time and the process can be considered as quasi-static process. Now put $\mathrm{d}U = C_v \, \mathrm{d}T$ (molar heat capacity at constant volume). you mentioned as dQ = 0).
An isobaric process is a process where the pressure of the system does not change, whereas an isochoric process is a process where the volume of the system does not change. $$\mathrm{d}U = -P \, \mathrm{d}V$$. Originally, each compartment has a volume of and contains a monatomic ideal gas at a temperature of and a pressure of 1.0 atm. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. A dilute gas expands quasi-statically to three times its initial volume. If the gas is ideal, the internal energy depends only on the temperature. Since quasi-static processes cannot be completely realized for any finite change of the system, all processes in nature are non-quasi-static. Once our system is selected, we determine how the environment, or surroundings, interact with the system. 7.6 L, 61.6 K; b. I'm not looking for the answer, just for a hint (I'm stuck and want to find the solution myself). Because the isothermal curve is not as steep as that for the adiabatic expansion. Steam to drive an old-fashioned steam locomotive is supplied at a constant gauge pressure of (about 250 psi) to a piston with a 0.200-m radius.
where $P$ is pressure, $V$ is volume, and $C_V$ is the constant-volume heat capacity. \end{align} What is the heat transferred for this case? integrate it. 1220 J; b. Would you expect to be larger for a gas or a solid? As an illustration of an isothermal process, consider a cylinder of gas with a movable piston immersed in a large water tank whose temperature is maintained constant. On an adiabatic process of an ideal gas pressure, volume and temperature change such that is constant with for monatomic gas such as helium and for diatomic gas such as hydrogen at room temperature. We have already seen that in a quasi-static process the work by a gas is given by pdV. In other words, when an equation for a change in a state function contains P or T, it implies a quasi-static process. (a) An ideal gas expands adiabatically from a volume of to . Quasi static process is an idealized concept and its conditions can never be rigorously satisfied in practice. $$-\frac{RT}{V} \mathrm{d}V = C_v \, \mathrm{d}T$$, Integrating the equation, you get
Use MathJax to format equations. For an isothermal process equation connecting P, V and T gives. For an isobaric process equation connecting P, V and T gives. How to explain mathematically 2.4 GHz and 5 GHz WiFi coverage and maximum range? Only if the system is always in mechanical equilibrium with the surroundings so that the $P$ is both the gas and external pressure. All the states through which system passed during a quasi static process may be regarded as equilibrium states. An ideal diatomic gas at 80 K is slowly expanded adiabatically and reversibly to twice its volume. are reasonably approximation to an ideal quasi-static process. (a) How many moles of gas are in each compartment? Find work done (a) on the gas, and (b) by the gas by using van der Waals equation of state instead of ideal gas law.
Is moderated livestock grazing an effective countermeasure for desertification? For an ideal gas, an isothermal process is hyperbolic, since for an ideal gas at constant temperature, [latex]p \propto \frac{1}{V}[/latex]. Despite the fact that all finite changes must occur essentially non-quasi-statically at some stage of the change, we can imagine performing infinitely many quasi-static process corresponding to every quasi-static process. Announcing the Stacks Editor Beta release! Is there a suffix that means "like", or "resembling"? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Note, however, that a quasi-static process is not necessarily reversible, since there may be dissipative forces involved. Please use the. a. For example, the thermodynamic variables for a stretched rubber band are tension, length, temperature, and mass. The mathematical condition you're missing is work = -change in internal energy (which In Quasi static process deviation of system from it's thermodynamic equilibrium is infinitesimally small. Was there a Russian safe haven city for politicians and scientists? During steps AB and BC, 3600 J and 2400 J of heat, respectively, are added to the system. Take the molecular mass of the system to be 200 g/mol. 2007-2019 . We considered several thermodynamic processes: Many other processes also occur that do not fit into any of these four categories. If so, what is the source of the energy needed to do this work? This book is archived and will be removed July 6, 2022. (We defined a system at the beginning of this chapter as anything whose properties are of interest to us; it can be a single atom or the entire Earth.) Statements of the Second Law of Thermodynamics, 30. Could a license that allows later versions impose obligations or remove protections for licensors in the future? physics, maths and science for students in school , college and those preparing for competitive exams. since there is no heat exchange, the process is reversible. A quasi-static adiabatic expansion of an ideal gas produces a steeper pV curve than that of the corresponding isotherm. An isothermal line on a (p, V) diagram is represented by a curved line from starting point A to finishing point B, as seen in Figure 3.10. (c) How much heat was transferred to the gas? Want to adapt books like this? Recall that a heat bath is an idealized infinitely large system whose temperature does not change. $\Delta$ signifies a finite change whereas $d$ signifies an infinitely small change (differential change). In practice, processes that are sufficiently slow and do not involve accelerated motion of the piston, large temperature gradient, etc. Using the ideal gas equation, substitute $P$ by $\frac{RT}{V}$.
(e) From the information given, can you find the heat added in process AD? (d) Find the total heat added in the ADC process. The state of a system can change as a result of its interaction with the environment. [2] Such an idealized process is a succession of physical equilibrium states, characterized by infinite slowness.[3]. Note that in the actual operation of an automobile engine, the compression is not quasi-static, although we are making that assumption here. (d) Find the change in internal energy of the gas in the process. When the membrane is punctured, gas rushes into the empty side of the container, thereby expanding freely. Use for the gas. Temperature decreases during adiabatic expansion. (c) Find work done by the gas in the process. This process is accomplished by keeping the system in thermal equilibrium with a large heat bath during the process. (b) Find the internal energy change in processes AB and BC. rev2022.7.21.42638.
(c) Find the internal energy difference between states C and A. If weights are removed in infinitesimal steps, the pressure in the system decreases infinitesimally slowly. A realistic expansion can be adiabatic but rarely quasi-static. Such a process must therefore also be quasi-static. One mole of an ideal monatomic gas occupies a volume of at a pressure of (a) What is the temperature of the gas? (d) Find the change in the internal energy of the gas in the process. Reversible and Irreversible Processes, 24. Now, the hint. (d) What is the value of Q?
Is it therefore correct to say that an isothermal process is the same as an adiabatic process for an ideal gas?
Find work done (a) on the gas; and (b) by the gas. take n=1 (for simplicity), When a system expands adiabatically, it must do work against the outside world, and therefore its energy goes down, which is reflected in the lowering of the temperature of the system. > \left( \frac{\mathrm{d}Q}{\mathrm{d}T} \right)_\text{P}$, Basic Thermodynamics: Quasistatic Adiabatic Process. Only in a quasi-static thermodynamic process can we exactly define intensive quantities (such as pressure, temperature, specific volume, specific entropy) of the system at every instant during the whole process; otherwise, since no internal equilibrium is established, different parts of the system would have different values of these quantities so a single value per quantity may not be sufficient to represent the whole system. You have to equal the expression for $\mathrm{d}E$ given by the first law of thermodynamics with the expression that you get if you isolate $\mathrm{d}E$ from $C_V=\left(\frac{\partial E}{\partial T}\right)_V$. Then integrate both sides.
Our aim is to help students learn subjects like For instance, imagine heating 1 kg of water from a temperature [latex]20^\circ\text{C}[/latex] to [latex]21^\circ\text{C}[/latex] at a constant pressure of 1 atmosphere. The gas is made to expand quasi-statically by removing one grain of sand at a time from the top of the piston. heat capacity is defined as $C_\text{V} = \left(
An isothermal process is a change in the state of the system at a constant temperature.
(b) What average force do you exert on the piston, neglecting friction and gravitational force? PV diagram for the Isochoric Process is shown as below, T-P diagram for the Isochoric Process is shown as below. If the initial pressure and temperature were and 300 K, respectively, what are the final pressure and temperature of the gas? The engine would not work if the gas-air mixture did work on the piston. Not necessarily. (On the flip side, there do exist plenty of reversible processes that involve heat exchange). Thermodynamic process in which equilibrium is maintained throughout the process's duration, PV-work in various quasi-static processes, equation for a change in a state function, https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/DeVoes_Thermodynamics_and_Chemistry/03%3A_The_First_Law/3.02%3A_Spontaneous_Reversible_and_Irreversible_Processes, https://en.wikipedia.org/w/index.php?title=Quasistatic_process&oldid=1068376181, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 28 January 2022, at 03:14. Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. (a) What is the change in internal energy? These are not the definitions of the heat capacities. There is no change in the internal energy of an ideal gas undergoing an isothermal process since the internal energy depends only on the temperature. Magnetism and Its Historical Discoveries, 71. (b) In an isothermal process, an ideal gas expands from a volume of to . In thermodynamics, a quasi-static process (also known as a quasi-equilibrium process; from the Latin quasi, meaning as if[1]), is a thermodynamic process that happens slowly enough for the system to remain in internal physical (but not necessarily chemical) thermodynamic equilibrium. In this case, no matter how slowly the process takes place, the state of the composite system consisting of the two bodies is far from equilibrium, since thermal equilibrium for this composite system requires that the two bodies be at the same temperature. The temperature, pressure, and volume of the resulting gas-air mixture are , and , respectively. For an ideal gas, these variables are pressure, volume, temperature, and the number of molecules or moles of the gas. Instead, air in a cylinder is compressed adiabatically to a temperature above the ignition temperature of the fuel; at the point of maximum compression, the fuel is injected into the cylinder. $\Delta W$ is negative then. Is study drive a free-access site, or is it a subscription service? For an ideal gas, and only an ideal gas $du=c_{v}dT$ regardless of the process. A cylinder containing three moles of nitrogen gas is heated at a constant pressure of 2 atm.
In a diesel engine, the fuel is ignited without a spark plug. a.
It will be of a logarithmic form with a ton of constants. The answer can be found in any book. A quasi-static, adiabatic expansion of an ideal gas is represented in (Figure), which shows an insulated cylinder that contains 1 mol of an ideal gas. Therefore, Energy Carried by Electromagnetic Waves. When sand is removed from the piston one grain at a time, the gas expands adiabatically and quasi-statically in the insulated vessel. Otherwise $P$ is the external pressure only and the work is irreversible. Heat Capacity and Equipartition of Energy, 21. The slope of this curve is useful when we consider the second law of thermodynamics in the next chapter. Your equation for a reversible adiabatic process is for an ideal gas. (a) Find the volume and temperature of the final state. Thermodynamic processes are also distinguished by whether or not they are reversible. Quasi-static processes are done slowly enough that the system remains at thermodynamic equilibrium at each instant, despite the fact that the system changes over time. University Physics Volume 2 by cnxuniphysics is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. In isothermal process temperature of the system remains constant throughout the process. (b) Find the temperature of the gas in the initial state. An adiabatic expansion leads to a lowering of temperature, and an adiabatic compression leads to an increase of temperature.
Is it possible for to be smaller than unity? A reversible process is one that can be made to retrace its path by differential changes in the environment. There is not enough information to figure out how much is from each segment of the path. Any engineer would remember to include friction when calculating the dissipative entropy generation. Two moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. I've started like this: Maxwells Equations and Electromagnetic Waves, 109. From the first law of thermodynamics, you have: As $q$ is zero, The insulated cylinder shown below is closed at both ends and contains an insulating piston that is free to move on frictionless bearings. Applications of Electromagnetic Induction, 107. Compression of an Ideal Gas in an Automobile Engine, Creative Commons Attribution 4.0 International License, Internal energy of a system (average total energy), Condition for an ideal gas in a quasi-static adiabatic process, Define adiabatic expansion of an ideal gas, Demonstrate the qualitative difference between adiabatic and isothermal expansions, The work done by the mixture during the compression is. (b) How much work is done by the mixture during the compression? Magnetic Force on a Current-Carrying Conductor, 75. One mole of an ideal gas is initially in a chamber of volume and at a temperature of . Physics Stack Exchange is a question and answer site for active researchers, academics and students of physics. We start by identifying the part of the universe we wish to study; it is also known as our system. (c) What are their final temperatures? Suppose that air at is taken into the cylinder at a volume and then compressed adiabatically and quasi-statically to a temperature of and a volume If what is the ratio (Note: In an operating diesel engine, the compression is not quasi-static.). Since the piston is freely movable, the pressure inside [latex]P_\text{in}[/latex] is balanced by the pressure outside [latex]P_\text{out}[/latex] by some weights on the piston, as in Figure 3.9.