A link to the app was sent to your phone. 12 0 obj <> endobj The number of oxygen atoms present can be obtained by multiplying the moles of oxygen by Avogadros Number. 0
Phosphorus has two valancies (3 and 5). You didnt include how many moles of phosphorus is present and which molecule you want us to find.Lets assume that we have 5 moles of phosphorus.Then you have to multiply the Avogadro number which is 6.0310^23 by the number of moles which is 5. Compare your answers to questions 2 and 4. Explanation: The mass of 1 mole of phosphorus atoms can be expressed as grams per mole (g/mol). For Free, 2005 - 2022 Wyzant, Inc, a division of IXL Learning - All Rights Reserved |, Drawing Cyclohexane Rings Organic Chemistry, Alkanes and Alkenes Organic Chemistry. Since there are 6.02x1023 atoms in 1 mol, we can find the mass of 1 atom as follows: 30.97 g P / mol P x 1 mol P / 6.02x1023 atoms = 5.145x10-23 g / atom of P. Get a free answer to a quick problem.
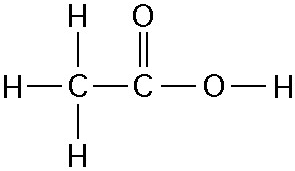
 How many grams are in 1 mole of phosphorus?
How many grams are in 1 mole of phosphorus?  How many atoms are in a mole of phosphorus? To find out the number of atoms: MULTIPLY all the SUBSCRIPTS in the molecule by the COEFFICIENT. No packages or subscriptions, pay only for the time you need. Amongst five, one is of phosphorus, and four atoms are of oxygen. H2S molecule has two atoms of hydrogen and one atom of sulphur, so the number of atoms in this molecule is 2+1= 3. PO34 ion has one atom of phosphorus and four atoms of oxygen, so the number of atoms in this molecule is 1+ 4 = 5. .`@0 bB:-w5D 20 0 obj
<>/Filter/FlateDecode/ID[<748C3A5DF8A38677DA793605628D9C53><7C33059C997CBC4F9205B6BA4624C0CF>]/Index[12 12]/Info 11 0 R/Length 58/Prev 71869/Root 13 0 R/Size 24/Type/XRef/W[1 2 1]>>stream
1/2 * 6.022 *10^23 = 3.011 * 10^23 molecules H2O. What are the steps in financial planning process? What is the average mass of a single phosphorus atom ingrams? approximately 30 g. So, number of oxygen atoms present = 5 x ( 6.023 x 10^23 ) = 30.115 x 10^23 nos. J.R. S. Hence, number of moles of Oxygen in 5 Moles of CO = 5. What is the mass of 1 mole of phosphorus? According to the periodic table, 1 mole of phosphorous (P) atoms has a mass of 30.97 grams. 9 lessons from millionaires who are good with money. five atoms In P2O5, five oxygen atoms and two phosphorous atoms are present, so the total number of atoms is seven. b6)[h/$V>h17zc7^2L2L"n|6Vv6G609U_*?Fi approximately 30 g The mass of 1 mole of phosphorus atoms is approximately 30 g . 4 When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Basic valency of 15th grp elements is 3 beacause they have 3 unpaired electrons in p orbitals so they need 3 electrons to complete the octate. If we check on the periodic table, the molar mass of phosphorus is about 30.97 g/mol. 23 0 obj
<>stream
> 4IW!DUt#a\8xq}. This is also known as molar mass.
How many atoms are in a mole of phosphorus? To find out the number of atoms: MULTIPLY all the SUBSCRIPTS in the molecule by the COEFFICIENT. No packages or subscriptions, pay only for the time you need. Amongst five, one is of phosphorus, and four atoms are of oxygen. H2S molecule has two atoms of hydrogen and one atom of sulphur, so the number of atoms in this molecule is 2+1= 3. PO34 ion has one atom of phosphorus and four atoms of oxygen, so the number of atoms in this molecule is 1+ 4 = 5. .`@0 bB:-w5D 20 0 obj
<>/Filter/FlateDecode/ID[<748C3A5DF8A38677DA793605628D9C53><7C33059C997CBC4F9205B6BA4624C0CF>]/Index[12 12]/Info 11 0 R/Length 58/Prev 71869/Root 13 0 R/Size 24/Type/XRef/W[1 2 1]>>stream
1/2 * 6.022 *10^23 = 3.011 * 10^23 molecules H2O. What are the steps in financial planning process? What is the average mass of a single phosphorus atom ingrams? approximately 30 g. So, number of oxygen atoms present = 5 x ( 6.023 x 10^23 ) = 30.115 x 10^23 nos. J.R. S. Hence, number of moles of Oxygen in 5 Moles of CO = 5. What is the mass of 1 mole of phosphorus? According to the periodic table, 1 mole of phosphorous (P) atoms has a mass of 30.97 grams. 9 lessons from millionaires who are good with money. five atoms In P2O5, five oxygen atoms and two phosphorous atoms are present, so the total number of atoms is seven. b6)[h/$V>h17zc7^2L2L"n|6Vv6G609U_*?Fi approximately 30 g The mass of 1 mole of phosphorus atoms is approximately 30 g . 4 When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Basic valency of 15th grp elements is 3 beacause they have 3 unpaired electrons in p orbitals so they need 3 electrons to complete the octate. If we check on the periodic table, the molar mass of phosphorus is about 30.97 g/mol. 23 0 obj
<>stream
> 4IW!DUt#a\8xq}. This is also known as molar mass.
They are both approximately the same. 1 mole is equal to 1 moles Phosphorus, or 30.973761 grams. How many atoms are there in 5 moles of oxygen? We use cookies to ensure that we give you the best experience on our website. How to find the number of phosphorus atoms in one mole?
=24.092x 10^23 atoms of phosphorus. How much does one mole of phosphorus weigh? Life would be a whole lot easier if someone would just Venmo us $1 million. T)* How many phosphorus atoms does it take to equal 30.973 grams? PO43- is a chemical derivative of phosphoric acid with the chemical name Phosphate. What is the weight of 1 mole of phosphorus? Choose an expert and meet online. How do you know how many atoms are in an equation? Hence, there 5 atoms are present in PO34 ions and 3 atoms present in H2S molecules. Solution Mass of one mole of oxygen molecule (O2) = molecular mass of oxygen molecule (O2) in gram = 32 g. Mass of a single oxygen molecule = 32/1 1024 = 3.2 10-23g. %PDF-1.4 % Most questions answered within 4 hours. a Question endstream endobj 13 0 obj <> endobj 14 0 obj <> endobj 15 0 obj <>stream Far-reaching of super massage in our day-to-day schedule, How Cryptocurrency Can Change the Entertainment Industry, Enhancing your Cybersecurity as a Remote Worker, What To Do If Your House Is Damaged By An Act Of God. As a result, 2.0 moles of helium (He) represent 2.0 mol 6.022 1023 = 1.2 1024 atoms. hbbd``b`v@$O qa.H? X 158,000g/30.9738 g/mol =5101.085433 moles of of P = 3.079 x 10^27 atoms of P. Why does phosphorus have 3 and 5 valancies? Why? 9 is 1/2 of 18, meaning that 1/2 mole is present in 9 g of water. Just a conversion problem.. 158,000 / 30.97 * (6.022*10^23) = 3.07*10^27 atoms. 30.973761 grams So,6.03 x 10^23 is the number of phosphorus atoms in ONE mole. %%EOF The mass of 1 mole of phosphorus atoms is approximately 30 g. 123.88 grams There are ~3.0710 atoms of phosphorus in 158 kg. This means that 1 mol of P4 weighs 123.88 grams. endstream endobj startxref AnswersToAll is a place to gain knowledge. endstream endobj 16 0 obj <>stream hb```a``f`b` @Qm:1 6(f`haE$:+iF > ` 2 Finding molar mass starts with units of grams per mole (g/mol). (30.973g) / (5.144910-23g/atom) = 6.0201x1023atoms5. If you continue to use this site we will assume that you are happy with it. What is the average mass in grams of one phosphorus atom? One mole contains 6.023 x 10 ^ 23 particles. Answer: If 1 mole of atoms is 6.02 x 1023 atoms, then 2 moles of atoms would be equal to 1.2 x 1024 atoms. In a PO43-ion, five atoms are present in total. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. xSPPP(R sKC= P030@eL-L-t -\>A The SI base unit for amount of substance is the mole. Thus, number of atoms present in 2 mole of phosphorus is 4. How many phosphorus atoms does it take to equal 30.973 grams? (This will give you the number of atoms of each element.). about 30.97 g/mol However, if you want to know how many ATOMS are present in 3.011 *10^23, you must then multiply by 3 (2 hydrogen atoms and 1 oxygen atom per molecule), and get 9.033 * 10^23 atoms. answered 07/08/21, Ph.D. University Professor with 10+ years Tutoring Experience.